Abstract
Introduction:
Angiogenic factors (AF) are the prototypic growth factors required for wound healing, and are linked to the immune response after tissue injury. Angiogenesis and inflammation together influence recovery from organ damage after hematopoietic stem cell transplantation (HCT), as well as contribute to the risk for complications such as acute graft-versus-host disease (aGVHD). We have recently described alterations in circulating angiogenic factors occurring at the onset of aGVHD post-HCT (BBMT 2015 21(6):1029), with dysregulation in AF associated with tissue repair versus those reflective of tissue damage. We hypothesized that the AF-inflammatory signature identified in the plasma in severe aGVHD could be linked to the gene expression of circulating donor cells after HCT. We performed transcriptomal analysis of changes in the peripheral blood immune cells and correlated with plasma AF levels in order to understand if the circulating donor cells might contribute to the AF profile.
Methods:
Peripheral blood samples from 3 patients (uncomplicated without aGVHD, steroid responsive aGVHD, and steroid refractory aGVHD) were compared to a healthy stem cell donor. Total RNA was derived from whole blood samples, and samples were amplified and labeled using MessageAMP Premier RNA Amplification kit (Ambion). Microarray analyses were performed using Human PrimeView GeneChip arrays (Affymetrix). Plasma AF were quantified using a multiplex angiogenesis array (Millipore).
Results:
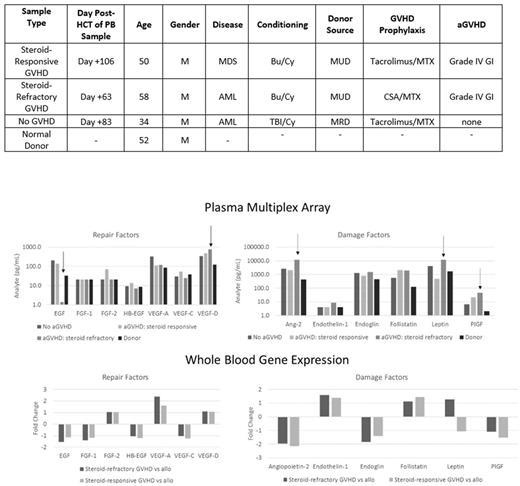
Patients were matched as able according to age, gender, conditioning intensity, donor characteristics, and disease (Table). All patients were 100% donor chimeras and without active infections at the time of blood draw. Compared to HCT donor, all HCT recipients had increased circulating levels of Angiopoietin-2 (Ang-2), endoglin, follistatin, placental growth factor (PlGF), and vascular endothelial growth factor (VEGF) (Figure). However, in steroid refractory aGVHD, levels of epidermal growth factor (EGF) were markedly lower compared to donor, steroid-responsive, and uncomplicated HCT recipients (1.4 pg/ml vs 32.4, 137.1, and 204.8 pg/ml respectively), while levels of VEGF-D, Ang-2, leptin, and PlGF were highest in steroid refractory aGVHD. In microarray analysis (Figure), steroid refractory aGVHD was again associated with a lower gene expression of EGF, but higher expression of only VEGF-D and leptin. Gene expression of Ang-2 and PlGF was not higher in hematopoietic cells, despite higher plasma levels, in steroid-refractory aGVHD, leading to the hypothesis that these growth factors may be predominantly host-derived. Interestingly, we found higher transcript expression of IFN-gamma, comparing steroid refractory versus steroid responsive aGVHD, along with a lower transcript expression of inflammatory cytokines TNF, IL-6, and IL-1B.
Conclusions:
The plasma profile of steroid-refractory aGVHD (low EGF, and high VEGF-D, Ang-2, leptin, and PlGF) appears to be only partially explained by donor hematopoietic cell gene expression. Gene expression of Ang-2 and PlGF are lower in steroid-refractory aGVHD, suggesting that the plasma elevations in these factors might be predominantly host-derived. Ang-2 likely reflects host endothelial tissue damage as previously described, while the PlGF response to aGVHD requires further investigation. The elevation in several damage-associated factors in the plasma and transcriptome in a healthy HCT recipient may reflect normal adaptation of donor cells to a new host environment. These results are hypothesis generating and mechanistic studies aimed at understanding and manipulating the dysregulated system of AF and downstream signaling pathways are indicated, with the goal of ameliorating severe aGVHD and facilitating tissue repair, without excess immunosuppression.
Holtan: Incyte: Other: One-time advisory board member.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal